Find out how State and Federal IT systems are working together to manage data reporting for Covid-19 vaccine production and distribution.
— Formaspace
AUSTIN, TEXAS, UNITED STATES, January 28, 2021 /ЕИНПрессвире.цом/ — President Biden shared an encouraging note this week when he spoke to reporters about increasing the incoming administration’s daily Covid-19 vaccination goal from 1 million shots to 1.5 million shots per day.
This newfound optimism is tempered, however, by the continued rocky rollout of the vaccination program across the nation.
In the face of unprecedented demand (and limited vaccine supplies), state and local public health officials are scrambling to get vaccine shots into people’s arms as soon as possible.
The process has been frustrating all around. Many Americans who rejoiced in getting one of the highly sought after vaccination appointments were crushed to find their appointments canceled because the federal government had missed its forecast for available vaccine deliveries to the states.
Even incoming CDC director, Dr. Rochelle Walensky, admitted there is a major problem. Speaking to Fox News, she said, “I can’t tell you how much vaccine we have, and if I can’t tell it to you, then I can’t tell it to the governors, and I can’t tell it to the state health officials… If they don’t know how much vaccine they’re getting, not just this week but next week and the week after, they can’t plan. They can’t figure out how many sites to roll out, they can’t figure out how many vaccinators that they need, and they can’t figure out how many appointments to make for the public.”
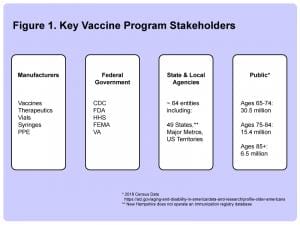
Who Are The Primary Stakeholders In The Nation’s Covid-19 Vaccination Program?
To the casual observer, it may seem incomprehensible that we were able to develop, test, and authorize two Covid-19 vaccines from Moderna and Pfizer BioNTech for emergency use in less than a year but, so far, the process of managing the supply chain—from production to delivering shots into people’s arms— has been marked by frustrating delays.
Зашто је то случај?
Is it a redux of the 2013 healthcare.gov website rollout that crashed and burned in the days after it was launched with great fanfare by President Obama?
Unfortunately, the analogy has some merit.
As was the case with Obamacare, IT system architects working on system integration projects for the Covid-19 vaccination efforts across the country are facing a similar challenge of how to glue together a large number of decentralized database systems operated by distinct stakeholders, including the Фармацеутска индустрија manufacturers, at least five major federal government departments, approximately 64 state and local vaccination tracking systems, as well as approximately 255 million Americans over the age of 18.
If you are looking for your TLDR moment, this is it.
The sheer number of decentralized database systems handling protected health information (PHI) across multiple levels of government combined with the challenges posed by manufacturing the first-ever approved mRNA vaccines (which must be maintained at incredibly low temperatures and require two injections several weeks apart for each patient) is the kind of nightmare scenario that would wake up the average IT systems engineer in the middle the night screaming—with good reason!
The only good news in this complicated situation is that this program is single-payer; in other words, the federal government is footing the bill, and thus, there is no need to bring in the added complications that bedeviled the healthcare.gov rollout, such as calculating patient subsidies, insurance plan coverage, and CMS reimbursements.
Structure Of The “Operation Warp Speed” Vaccination Development Effort
Let’s take a deeper dive into each of these systems, starting with the structure of “Operation Warp Speed” (or OWS) that was launched in March 2020.
The two goals of the OWS program were to
– provide strong, direct financial backing from the federal government for any pharmaceutical company that showed a promising candidate for Covid-19 vaccines or therapeutics
– fund large-scale production of vaccine and therapeutic candidates (as well as critical items such as storage vials, syringes, PPE, etc.) in parallel to the research and development programs. In other words, the federal government foots the bill for ramping up production even before knowing whether clinical trials would pan out or not.
The federal government engaged McKesson as the chief central distribution contractor – a sensible choice since they had a track record since 2006 of managing the federal distribution of more than 1 50 million doses for influenza, chickenpox, and MMR vaccines.
OWS also brought on Palantir to provide “big data” supply chain tracking, inventory management, and data forecasting tools. In keeping with the warp speed Star Trek theme, the Palantir implementation was christened “Tiberius,” after Starship Enterprise’s Capt. Kirk’s middle name.
The company, founded by billionaire PayPal co-founder Peter Thiel, has not been without controversy, particularly among privacy advocates, who argue that the company’s data collection efforts have gone too far. Nonetheless, Palantir successfully leveraged this and other federal contracts to take the company public in late 2020.
Extraordinary Efforts To Track Deep Freeze Temperatures For Moderna And Pfizer BioNTech MRNA-Based Vaccines
Two of the Covid-19 vaccines in development worried public health officials, the ones from Moderna and Pfizer BioNTech.
These concerns multiplied when it became apparent these two vaccines would be the first to receive FDA emergency authorization.
Julia Solodovnikova
Formaspace
+ КСНУМКС КСНУМКС-КСНУМКС-КСНУМКС
пошаљите нам е-маил овде
Посетите нас на друштвеним мрежама:
фацебоок
Twitter
ЛинкедИн
![]()